The decision to outsource your stability studies can be influenced by many different factors. The outcome is a success when collaborating with the right partner. Stability Storage Outsourcing is more successful if it is based on strategic decisions including core competence and cost efficacy considerations rather than being an emergency action prompted by short comings of long term planning of the overall demand for stability. In this article we focus on the reasons large manufactures, contract research organizations (CROs) and contract development manufacturing organizations (CDMOs) decide to outsource their stability storage.
Data from CROs and pharmaceutical companies reveal an increase in the amount of outsourcing of their stability needs. Stability testing is a requirement for all drug, cosmetic and nutraceutical products in order to detect the changes in potency, purity and identity as a result of environmental and processing factors. Whether this testing is done in-house or outsourced, these environmental conditions (see table) require a significant amount of time and resources to accommodate for the conditions and capacity needs required for stability. The specific conditions required for stability are not only based on a specific drug but also the stage of development, how the drug is intended to be used and in which markets the product is going to be released.
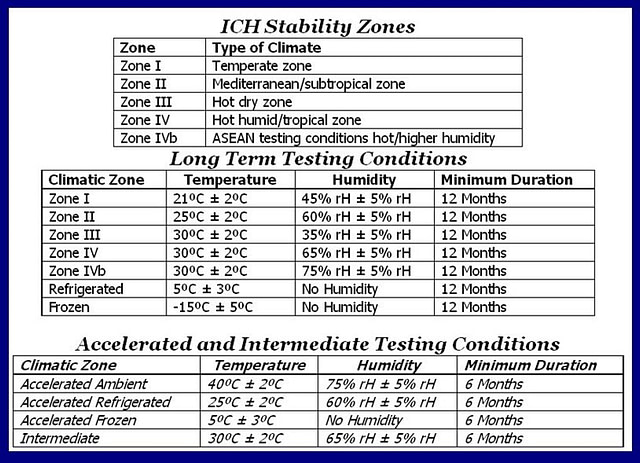
It can be a challenge for many organizations to conduct their own stability studies unless they have a lot of studies going through the pipeline. The resource management required for equipment and staff can add up because of the spaced-out time intervals of the pulls at 3, 6, 9, 12, 18 & 24 months. Some studies can last up to three years or longer. A contract storage facility can easily dedicate multiple personnel to these tasks to make sure every sample is double checked for accuracy and pulled on time per ICH guidelines. Outsourcing frees up personnel to be able to focus on the company’s core competencies such as research, manufacturing, clinical trials, marketing and license applications.
Capacity can also be an issue for many CROs and pharmaceutical companies to overcome. With the growing price of real estate, finding the footprint to place walk-in chambers can be difficult. Many companies will start off with small environmental chambers and soon realize they have outgrown them and require more capacity. Each addition requires more annual validations to be carried out and ensure each chamber is validated to a continuous monitoring system. The monitoring systems can also be costly to the capital budget ensuring they are CFR part 11 compliant.
Partnering up with a stability storage specialist can allow your lab to carry out tests that may not be currently on hand such as photostability test for stress testing under ICH Q1B guidelines or an intermediate condition of zone IVb at 30°C/75% RH. Retains storage is another capacity need that is often overlooked.
Choosing the right partner for your stability needs can be an easy process to ensure the contract lab is carrying out these studies according to ICH recommended guidelines with proper maintenance and validation procedures in place. Usually a QC representative will want to carry out a paper audit at a minimum and follow-up with an onsite audit to insure proper SOPs for sample management and stability are in place along with essential security, monitoring and a backup generator.
For further information and prices please contact us or call +44 (0) 1706 716710.




